What Engineers Should Know About ASTM B117 Salt Spray Corrosion Testing Today
ASTM B117 has been a core corrosion test method in laboratories and production lines for more than eight decades. The procedure is simple, stable, and widely recognized, which explains why it remains used by coating suppliers, hardware manufacturers, and metal finishing plants around the world. But engineering practices have changed. The industries that rely on long-term durability—automotive, aerospace, marine, and outdoor equipment—no longer view salt spray as a stand-alone indicator of real-world corrosion behavior. The purpose of the test, the way results should be interpreted, and its limitations are often misunderstood. Today’s engineers need a clearer view of what B117 can and cannot tell them.
The Role of ASTM B117 in Modern Corrosion Testing
ASTM B117 defines a controlled, continuous salt fog environment. Compressed air, atomization nozzles, a defined NaCl concentration, collection rate, and a stable temperature form a closed system that is highly repeatable. This repeatability is the core reason the method survives today. When two coatings, two plating lines, or two batches of treated parts need comparison, the stable environment makes differences easy to detect.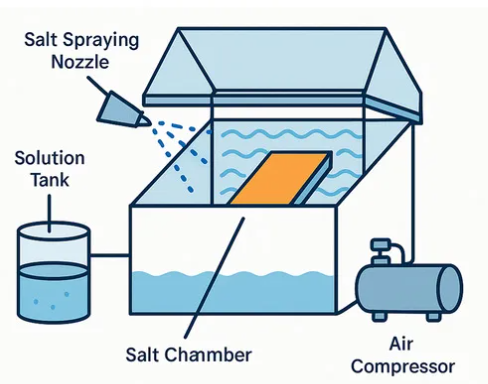
This also explains why factories still rely on salt spray for daily quality checks. Failures that originate from poor pretreatment, contamination, improper curing, or insufficient coating thickness typically reveal themselves within tens of hours. For mass-production environments, a method that gives fast feedback matters more than a method that perfectly mimics outdoor exposure.
Why Salt Spray Does Not Predict Service Life
The persistent misconception around B117 is the idea that a certain number of hours equals a certain number of years outdoors. No such conversion exists. The reason is that the corrosion mechanisms inside a salt spray chamber do not match the mechanisms that govern real environments.
Three factors make prediction impossible:
Single, continuous exposure
The chamber maintains constant humidity and constant chloride availability. In most outdoor conditions, corrosion is governed by wet-dry cycling. Salt deposits dry, crack, and re-dissolve repeatedly, which fundamentally changes corrosion kinetics. B117 does not include any drying phase.Different oxygen transport behavior
Outdoor steel corrosion depends heavily on oxygen diffusion through wet films. The static, saturated environment inside the chamber produces a different electrochemical balance.Material ranking may reverse
Coatings that perform well in continuous salt exposure may not resist UV, temperature cycles, or atmospheric pollutants. Conversely, coatings designed for outdoor durability—such as certain zinc-aluminum flake systems—do not necessarily excel in B117 despite excellent field performance.
Because of these mechanism differences, salt spray results cannot be extrapolated to years of outdoor performance, and standards bodies have never claimed otherwise. Engineers use B117 as a comparative tool, not a durability predictor.

Why the Test Is Still Useful
Although B117 cannot replicate real environments, it remains practical for many applications:
Detecting pretreatment or cleaning defects
Screening different coating formulations
Checking plating thickness uniformity
Monitoring process stability in high-volume production
Comparing two process routes during development
These tasks benefit from rapid feedback and stable test conditions. The simplicity of B117 creates high lab-to-lab consistency, something more complex corrosion tests struggle to achieve.
The Industry Shift Toward Cyclic Corrosion Testing
 Where real-world correlation matters—automotive body structures, engine bay components, marine hardware, aerospace fasteners—the industry is shifting toward cyclic corrosion tests. Methods such as ISO 16701, SAE J2334, GM9540P, VDA 233-102, and ASTM G85 introduce environmental transitions: salt deposition, drying, condensation, temperature cycling, and sometimes SO₂ contamination.
Where real-world correlation matters—automotive body structures, engine bay components, marine hardware, aerospace fasteners—the industry is shifting toward cyclic corrosion tests. Methods such as ISO 16701, SAE J2334, GM9540P, VDA 233-102, and ASTM G85 introduce environmental transitions: salt deposition, drying, condensation, temperature cycling, and sometimes SO₂ contamination.
These cycles reproduce the transitions that drive outdoor corrosion. For example, drying phases allow chloride crystallization and re-wetting, a mechanism absent in B117 but central to atmospheric corrosion. This explains why cyclic tests often align more closely with field exposure results.
For engineers designing products expected to last five to ten years outdoors, the question is no longer “how many hours of salt spray,” but “which test captures the corrosion mechanisms that matter for our application.”
How Engineers Should Use B117 Results
When used correctly, salt spray is a powerful diagnostic tool. The key is to treat results as relative indicators, not durability forecasts.
A practical engineering approach is:
Use B117 to verify whether a plating or coating process is consistent from batch to batch.
Compare different suppliers, pretreatment steps, curing profiles, or coating stacks.
Do not use B117 to provide customers with lifetime claims.
Combine B117 with cyclic corrosion testing or outdoor exposure when long-term behavior matters.
Document failures not only by hours but by failure mode: blistering, red rust formation, scribe creep, adhesion changes, or coating delamination.
This approach aligns with how major industrial sectors handle corrosion qualification today.

What Engineers Should Pay Attention to Right Now
The role of B117 is not disappearing. Instead, its function is narrowing. Engineers who work with coatings, metallurgy, component reliability, or regulatory compliance should focus on four current trends:
Salt spray is becoming a screening and process-control tool rather than a final qualification requirement.
Cyclic corrosion tests are becoming the primary method for products requiring real-world correlation.
Failure analysis in B117 now matters more than hours—how parts fail is more meaningful than time-to-rust.
Supply chains increasingly use B117 for consistency audits, especially when multiple vendors produce the same component.
Understanding these shifts helps prevent over-interpretation of test results and supports more accurate material decisions.
The Practical Takeaway for Manufacturers and Engineers
ASTM B117 remains valuable when its limitations are understood and respected. It is fast, stable, and well-suited for comparing treatments, verifying processes, and detecting manufacturing issues. It is not a method for estimating outdoor lifetime, and it cannot reproduce the complex cycles that drive real environmental corrosion.
For modern engineering applications, salt spray is one piece of a larger corrosion-evaluation framework. Used together with cyclic tests, field exposure, and failure-mode analysis, it continues to support reliable material selection and production-quality control.













